Researchers at North Carolina State University have created a new flexible nano-scaffold for rechargeable lithium ion batteries that could help make cell phone and electric car batteries last longer.
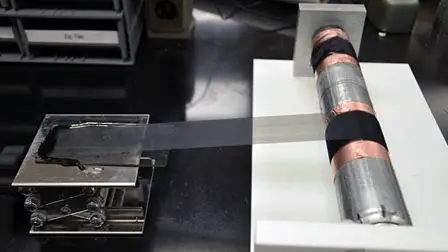
The research, published in Advanced Materials, shows the potential of manufactured sheets of aligned carbon nanotubes coated with silicon, a material with a much higher energy storage capacity than the graphite composites typically used in lithium ion batteries.
"Putting silicon into batteries can produce a huge increase in capacity – 10 times greater," said Dr. Philip Bradford, assistant professor. "But adding silicon can also create 10 times the problems."
One significant challenge in using silicon is that it swells as lithium ion batteries discharge. As the batteries cycle, silicon can break off from the electrode and float around (known as pulverisation) instead of staying in place, making batteries less stable. When the silicon-coated carbon nanotubes were aligned in one direction – like a layer of drinking straws laid end to end – the structure allowed for controlled expansion, so that the silicon was less prone to pulverisation.